Understanding the shelf life of your product plays a critical role in protecting your brand. Spoilage, as seen with bloating containers, off-odors, off-flavors, and texture defects, are some relatively common clues that a product considered to be “commercially sterile” is not meeting expectations. In general, sterility cannot be achieved without drastically changing the quality of the product, so these products are processed as commercially sterile.
Commercial sterility refers to the absence of microorganisms capable of growing in the food at normal non-refrigerated conditions at which the food is likely to be held during distribution and storage (Code of hygienic practice for aseptically processed and packaged low … 1993).
Traditionally, thermal processing technology has been widely applied to preserve food, as with canned foods. It now extends to Ultra-High Temperature (UHT) and aseptically filled products, including a wide range of products (i.e., plant-based beverages, broths, etc.) because of their convenience.
Commercial sterility testing methods are used to verify the efficacy of the process and its controls. Testing methods commonly used to demonstrate commercial sterility originate from the canning industry and are based on two main steps: pre-incubation in the final container and culture-based microbial detection of any growth. These methods are time-consuming and labor-intensive.

Several alternative rapid sterility testing methods are available such as BacT Alert, which provides a faster time to result and is sensitive to the presence of even low levels of microbial growth in food samples. It can detect various microorganisms, including bacteria, yeast, and mold. The automated method utilizes rapid sterility testing equipment, allowing continuous monitoring and tracking of samples, facilitating faster result reporting and efficient decision-making.
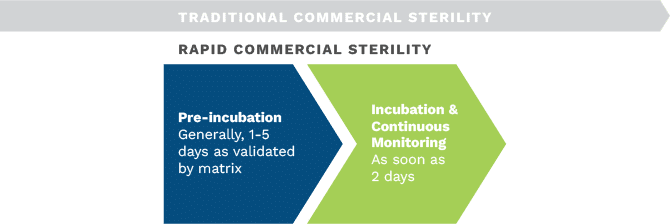
Our experts at the Silliker Food Science Center will work with you to review your product type and set up a validation before routine analysis that can provide rapid results.
If you have questions about our microbiology and rapid commercial sterility testing methods, please contact us!
References
Code of hygienic practice for aseptically processed and packaged low … (n.d.-b). https://www.nicd.ac.za/wp-content/uploads/2018/05/Code_of_Hygienic_Practice_for_Aseptically_Processed_and_Packaged_Low-Acid_Foods_CAC_RCP_40-1993.pdf