Highlights
- The New Normal for Food Labels
- Amended Regulations
- Implementation Timeline
What Is the New Norm for Food Labels?
Do you know how much potassium must be consumed when grabbing your first large cup of coffee in the morning? Are you able to identify all the sources of sugars in the strawberry jam and recognize how much sugar is to be consumed when spreading the jam onto your toast? When passing them along to your kids on a hot summer day, are you curious about what colors make fruity pops appear so bright and vivid?
Canadian consumers are now able to get this useful information on food labels.
- On the coffee label, the amount of potassium (mg) and % potassium is now declared in NFt.
- On the fruit jam label, all fruit purees and fruit juice concentrates are now grouped with added sugar and listed in the bracket with the term “sugars” in the list of ingredients. Percent total sugar is now declared with its amount (g) in NFt.
- On the fruit pops label, specific names of colors, such as brilliant blue and sunset yellow, are now declared individually in the list of ingredients.
What Are the Amended Regulations?
Health Canada published the amendments to Canadian Food and Drug Regulations related to nutrition regulations, list of ingredients, and food colors on December 14th, 2016. This amendment applies to all prepackaged food products sold in the Canadian market. It aims to provide consumers with much clearer, more useful, and easily comparable information at purchase.
The amended regulations mainly focus on two pieces of information on the food label: A list of Ingredients and a Nutrition Facts table.
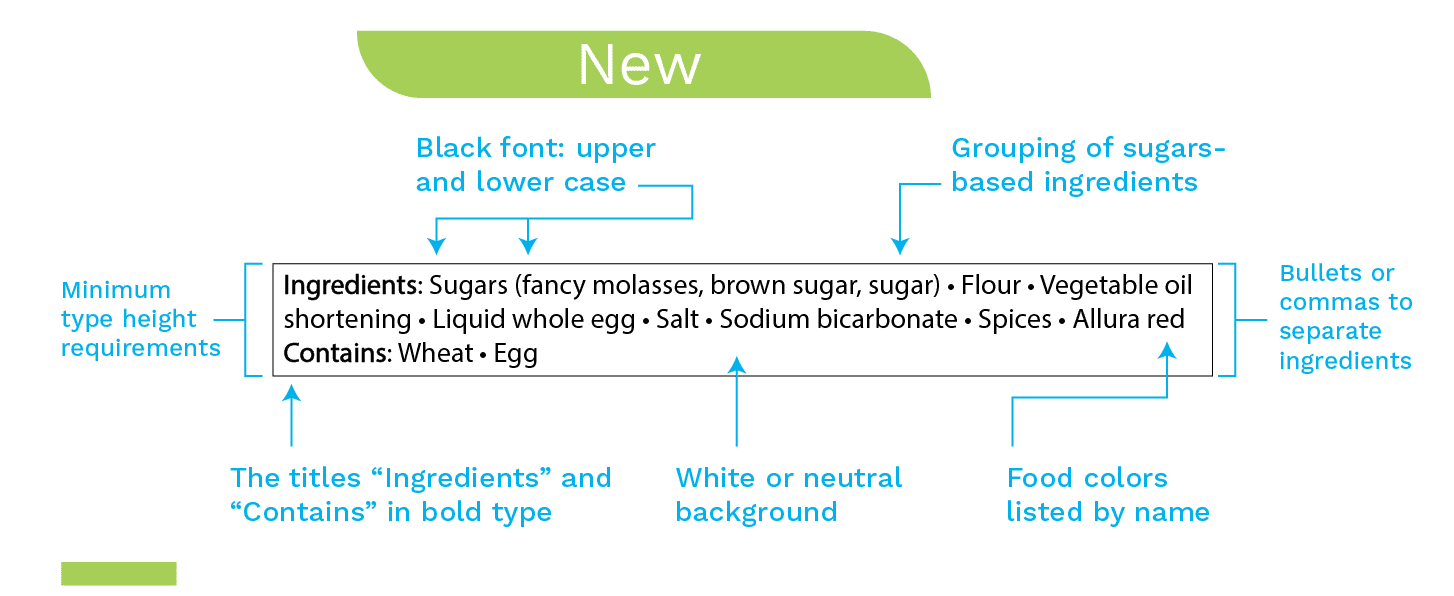
List of Ingredients
In addition to those listed above, changes have been made in the List of Ingredients. For example,
- Using black text in white or neutral backgrounds and adding a solid and same color line-box around the list of ingredients. This aims to make the list of ingredients easily located on the label.
- Using minimum type height (1.1mm font size) with identical minimum leading (2.5 mm) used in the list of ingredients. This aims to make the list of ingredients read more easily.
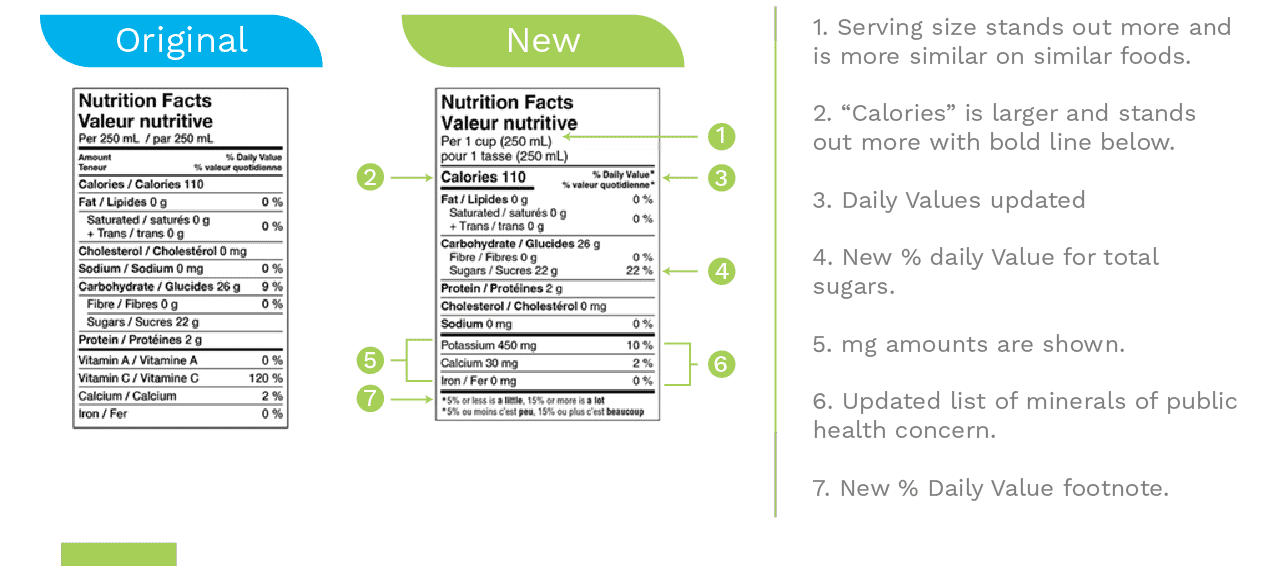
Nutrition Facts Table
In addition to those listed above, more changes have been made in NFt. For example:
- Use a larger font size for calories/serving size and a bold line under the calories to make the information more easily read.
- Adding a footnote: 5% or less is a little, and 15% or more is a lot aims to assist consumers in reading the percentage of each nutrient in serving the product.
- Consistent serving sizes for similar foods make the nutrition comparison much more straightforward.
What Is the Implementation Plan?
Health Canada originally gave the industry a 5-year transition period to implement these changes on food products sold in the Canadian market, with a deadline of December 14th, 2021. Due to the complexity of changes applied to the labels, as well as using up existing labels before applying the amended labels, Health Canada provides a detailed implementation plan for transition ending in December 2021 to assist the industry to be compliant with new regulations:
- Dec 14th, 2016 – Dec 14th, 2021 – existing labels with current or new labels with amended labeling requirements comply. CFIA focuses on education and compliance promotion of these amended labeling requirements.
- Dec 15th, 2021-Dec 14th, 2022 – amended labeling requirements for NFts and lists of ingredients are in effect. CFIA will continue to focus on education and compliance promotion of new labeling requirements.
- Dec 15th, 2022- Dec 14th, 2023 – labels on Canadian market products must comply with new labeling requirements unless individual companies have a detailed plan to comply by or before Dec 14th, 2023.
- After Dec 14th, 2023, labels on Canadian market products must comply with amended labeling requirements.
What Can Mérieux NutriSciences Do for You?
Mérieux NutriSciences offers comprehensive labeling compliance services, including developing regulatory-compliant labels for nutrition, ingredient statements, and allergen statements. We also offer complete nutrition labeling options and food label review services to meet Canadian regulations. Contact us to learn more about how we serve you better in labeling and nutrition compliance with Canadian Regulations.
