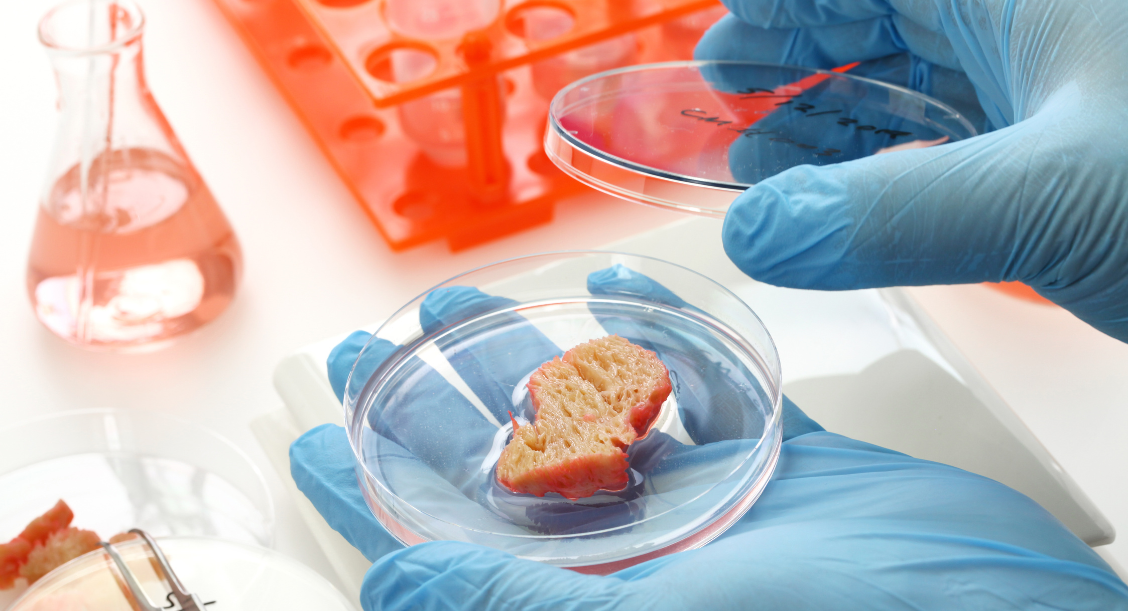
Our previous post highlighted the groundbreaking FDA approval of lab-grown meat in November. Fast forward, and now two companies that produce cell-cultured meat have recently received approval from the Food and Drug Administration (FDA) and the United States Department of Agriculture (USDA) to sell their products in the US. This is a significant milestone for the alternative protein industry, particularly for cell-cultured products [1]. These companies have met all the regulatory requirements, including obtaining a "No Question" letter from the FDA, approval for their product labels from the USDA, and the USDA Grant of Inspection (GOI). They are one step closer to bringing their cultivated meat products to the market, marking a historic achievement for both companies.
Cultivated from animal cells, cultured meat products could solve today's environmental sustainability challenges. Studies show that these alternative means of protein production could reduce the environmental carbon footprint, enhance resource efficiency, reduce antibiotic use, and promote diversity of protein sources [2-3].
What Are the Implications of These Approvals? What Lies Ahead of the Cultured Meat Industry?
The analytical methods required to analyze these products' safety, quality, and acceptability are minimal and must be harmonized. Validated official methods and authentic reference materials are necessities as these products enter the market. Our scientists at Mérieux NutriSciences have diligently followed the developments in this nascent industry. Our Environmental, Social, and Governance (ESG) goals and the commitments to support food safety and sustainability are at the forefront of all our initiatives.
How Can Merieux Nutrisciences Help?
Mérieux NutriSciences (MXNS) has become a partner for traditional and innovative food producers. We are ready to take this industry to the next level by collaborating with the stakeholders to address challenges from consumer acceptance to regulatory compliance. Our capabilities extend from nutrition and sensory profiling to process optimization and shelf-life extension.
.webp?width=234&height=87&name=46fea6f4-52fa-46f7-b3d7-6ad56b20134d%20(1).webp) To expand our expertise further and to fill the gaps in analytical testing methods, we have initiated collaboration with premier analytical organizations like the Association of Official Analytical Collaboration (AOAC International). We are organizing a scientific session and a discussion in this area at AOAC annual meeting and exposition in New Orleans on August 30. Our mission for these alliances is to develop validated official analysis methods with key stakeholders to guarantee the safety and acceptability of these novel products.
To expand our expertise further and to fill the gaps in analytical testing methods, we have initiated collaboration with premier analytical organizations like the Association of Official Analytical Collaboration (AOAC International). We are organizing a scientific session and a discussion in this area at AOAC annual meeting and exposition in New Orleans on August 30. Our mission for these alliances is to develop validated official analysis methods with key stakeholders to guarantee the safety and acceptability of these novel products.
With our analytical chemistry and microbiology expertise, we are committed to producing high-quality products that are acceptable and safe to our customers. With an unwavering commitment to customer safety at the core of our mission, we are poised to drive this industry to a promising future. Contact us to learn more about our analytical expertise!
.webp?width=234&height=87&name=46fea6f4-52fa-46f7-b3d7-6ad56b20134d%20(1).webp)
